



Auxins and the Pathways for Foliar Applications
Reprinted from: Hortus Plant Propagation from Cuttings, a guide to using plant rooting hormones by foliar and basal methods.
First Edition. 2009
By: Joel Kroin, President, Hortus USA Corp.
DOWNLOAD ARTICLE
Plant researchers had long known that plants produce chemicals that cause to divide and become roots. In 1934 Thimann and Went identified IAA (Indole-3-acetic acid), a rooting hormone, now called auxin. IAA is produced in the leaves of plants and is found in other plant parts. It is unstable and degrades rapidly in the presence of light and heat. More stable than IAA, the bio-simulators IBA (Indole-3-butyric acid) and (Naphthalene acetic acid) are the commercially used rooting compounds. IBA and NAA are stable and remain active for use by plants over a long time. IBA and NAA are known to induce plant cells to form adventitious roots. Of the three, IBA is the most useful auxin to propagate plants from cuttings.
The way that IAA, IBA and NAA are used by plants to induce root formation is not fully understood. The use of auxin treatments, and the
selection of the compounds, in rooting cuttings is not universal since some species react better than others to the treatment. The natural auxin, IAA, is bio-synthesized in fully developed and young leaves. It is also found in seeds. IAA is transported through the plant from
cell to cell in various parts of the plant, vascular cambium, pro-cambial strands and perhaps the epidermal cells.
Relative to root formation, some effects of the auxins have been observed:
• cell enlargement that likely increases root and stem length
• cell division that assists in root formation
• root initiation by inducing roots on stems and sometimes leaves
• apical dominance that sometimes effects the stem and leaf growth when using foliar applied auxins
• tropic responses (bending) that is sometimes noticed on tender leaves when using foliar applied auxins.
Auxins have other effects on plants:
• leaf senescence, delay of leaf drop
• leaf and fruit abscission, leaf and fruit drop
• fruit setting and growth
• promotes flowering in some plants like bromeliads
• growth of flower parts
• In some cases, the effect of excess auxin is to inhibit growth (1).
To induce root formation, early investigators and growers until today have applied the auxins to the basal end of the cuttings.
Dry compounds: Dry powder auxin compounds and solutions containing auxin were found to be useful. The auxins are blended with a carrier, usually talcum powder. Early users also mixed the auxins with powdered charcoal.
Liquid compounds: Common forms of the auxins are soluble in active solvents like alcohol. The solutions made with the auxins were first used in low concentration basal soaks.
Lanolin compounds: Not used today, auxin is mixed with lanolin, a natural material produced obtained from wool processing. The compound was applied to the basal end. In rare cases the lanolin compounds was applied to plant leaves. (8)
Using rooting solutions at low concentrations, early literature describes the basal long soak method. The basal end of the cuttings are immersed in the solution, at a few ppm auxin, for a few hours to several days. The method is successful on many kinds of plants. It has been used on soft herbaceous to woody ornamental plants. Later, the basal quick dip method was developed. The basal end of the cuttings are immersed in the auxin solution for a few seconds. The method uses auxin rates much higher than the basal long soak method. Alcohol as the solvent is need to make solutions of the auxins.
Early users found alcohol to cause phytoxicity to plant cells and case mortality to the cuttings. The water soluble salts of the auxins was not as well adopted. Therefore, the auxin solutions were used at low rates to allow use of low concentrations of the alcohol.
EARLY OBSERVATION:
leaf contribution to root formation.
In 1946, van Overbeek observed that the action of the natural plant rooting hormone in the leaves of plants is essential for plant cuttings to form roots at the basal ends. The rooting hormones are transported from the leaves to the basal end. The basal end is a wound sink point of cuttings.
Dutch Rhizopon researchers identified the stomata of leaves as a place to induce water soluble auxins into the plant’s vascular system to
stimulate root formation. In 1985 the Rhizopon techniques were introduced to growers. The methods are to spray the solution onto the
leaves, the Spray Drip Down, or to totally dip the cuttings into the solution, the total immerse method. Figure 1

FIGURE 1
FREE IAA PRODUCTION
The natural plant rooting hormone (auxin), IAA, is produced during the development of leaves. Hydrathodes are the water secreting glands
which develop in the leaf tips and later in the leaf lobes. As the leaves develop, the hydrathodes are the primary sites of free auxin production. Trochomes, the epidermal hair structure, and mesophyll, the storage cells between the epidermal layers of the leaf, are secondary sites. Figure 2
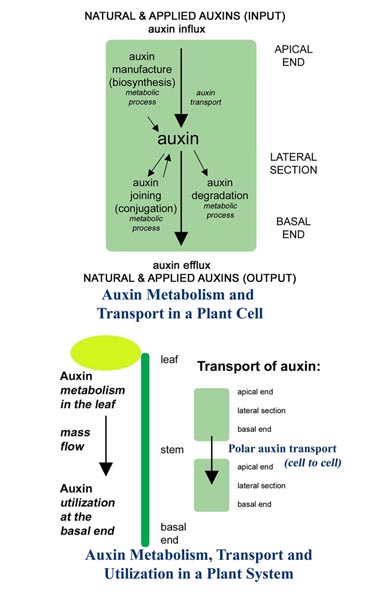
FIGURE 2
Free auxin production progresses from the elongating tip and continues downward along the expanding leaf blade margins. (3)
USEFUL AUXINS FOR FOLIAR APPLICATIONS
Both IBA and NAA have been found useful as single component applied auxins Of the three auxins, IBA has been most the most used for foliar application. Eigenraam at Rhizopon found that the combination of IAA and NAA, and IBA and NAA are effective on pot rose propagation.
CARRIER
As the natural fluid in the plant structure, water was selected by Rhizopon as the carrier for the plant rooting hormones for foliar applications of rooting hormone solutions. While alcohol is another solvent for rooting hormone compounds it was not selected. Alcohol can be phyto-toxic to plant tissues since it will dehydrate plant cells and cause the mortality; this phenomenon is commonly called ‘alcohol burns’.
INDUCTION POINT: THE STOMATA
Stomata are the minute openings in leaves that allow the flow of gases and fluids into the plant. The Stomata are protected, each by two guard cells. These cells cause the stomata to be open during normal room temperature. The stomata close when the plant is under stress, such as from heat or cold. Stomatal closure limits the water loss from the leaf area and to limit the size of the leaf area such as when curling. Auxins in a rooting solution, applied to open stomata, are taken into the plant’s vascular system. Air spaces under the guard cells and stomata serve to store the induced rooting solution, to be later translocated through the vascular system. Figure 3
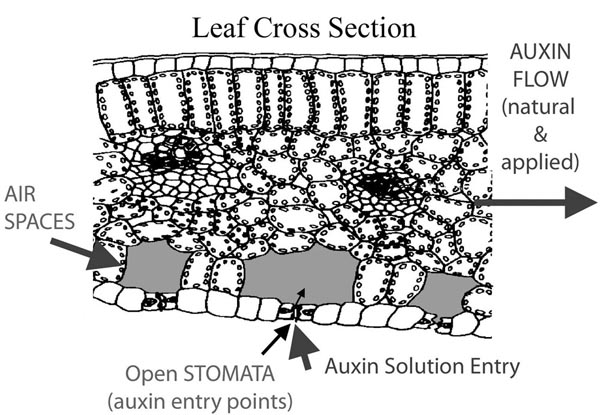
FIGURE 3
TRANSLOCATION: MASS FLOW
The auxins, in solution, are translocated rapidly by mass flow in the mature phloem. Auxin transport is strongly polar through cells and
tissues. They are transported cell to cell, from the leaf source downward toward the basal end of plant cuttings. The speed of motion, a few
centimeters per hour, is regulated by the plant. The flow is carrier dependent. For IBA and NAA the actual time of travel is not critical since
they are remain active a long time. The plant self regulates the use of the auxins. as it is needed, for root formation and other plant growth
functions. Studies of auxin transport have shown the velocity of IAA at 7.5 mm/hour, NAA at 6.7 mm/hour, and IBA at 3.2 mm/hour. Using
labeled auxins, IBA was reported the travel in polar transport; most of the IBA remained in the bases of the cuttings. (6) Figure 4
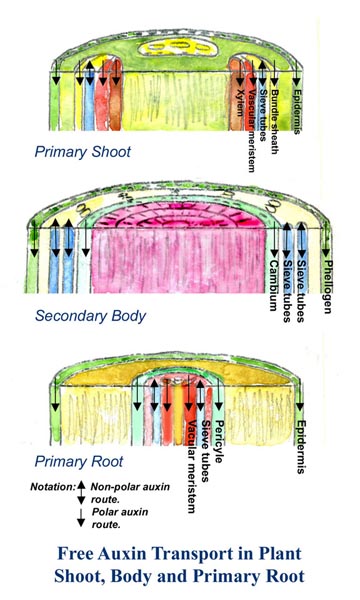
FIGURE 4
Two physiologically distinct, and spatially separated pathways, function to transport auxins over long distances through plants.
Polar transport (one way transport)
In the non-polar route, auxin is translocated rapidly by mass flow and other metabolites in the mature phloem. Transport is downward from
immature tissues close to the shoot apex toward the root tips. In solution, the auxin that is loaded into a mature phloem is translocated passively in the phloem sap to sink organs and tissues at the basal end where it is released. (4) Figure 4
In the non-polar route free auxin moves inside
• the primary shoot through the epidermis, bundle sheath, vascular
meristem, and the xylem.
• the secondary body through the phellogen, and the cambium.
• the primary root through the epidermis, pericycle, and vascular
meristem.
Non polar transport (two way transport)
In the non-polar route auxin moves up and down the sieve tubes. (5)
PLANT UTILIZATION OF IBA & IAA TO INDUCE ROOT FORMATION: IBA CONVERSION TO IAA
In some plant cuttings, IBA was shown to be slowly metabolized by the plant to IAA. The IAA and IBA co-factors serve as a time release growth regulator. IBA was found to be an endogenous, synthesized, constituent of various plants. Studies of auxin transport showed IAA was transported faster than IBA. Like IAA, IBA was transported mostly in a “basipetal direction”, polar transport. It has been claimed that easy to root, as opposed to difficult to root cultivars, have the ability to hydrolyze auxin conjugates during growth to time release free auxin which may induce and boost root initiation. This theory is supported by reports on increased level of free auxin in the bases of cuttings prior to rooting. Easy to root cultivars are also able to convert IBA to IAA which accumulated in the cutting bases. The higher rooting promotion of IBA was also ascribed to the fact that its level remained elevated longer than that of IAA even though IBA was metabolized in the tissue. (6) The metabolism of IBA was observed in hardwood cuttings of grapevine and olive. It was found that both plant species converted IBA to IAA as confirmed by chromatography. (7) Figure 5
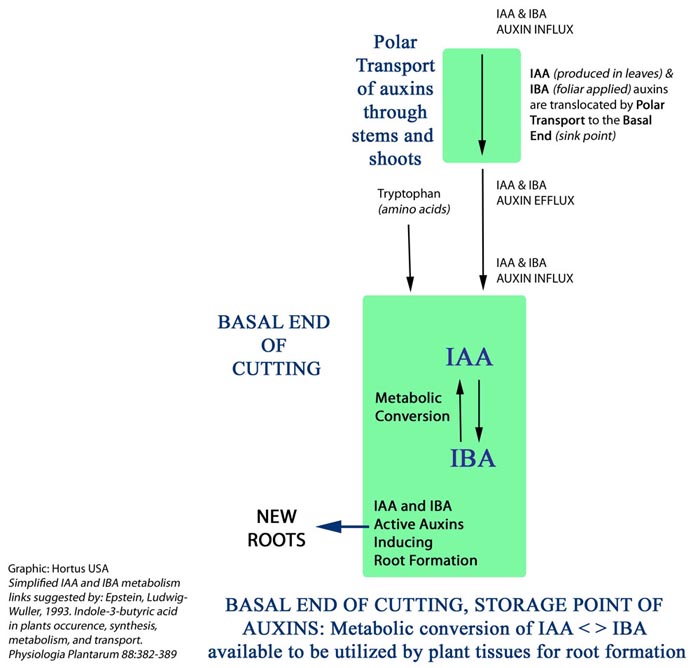
FIGURE 5
TIMING
Auxin solutions enter the stomata rapidly and are captured in the leaves and stems of the plants. The solutions are able to freely move down the leaves and stems toward the rooting site sink point. The speed of travel of the solution is influenced by plant variety, hydration and irradiance. Once the induced solution is in the plant system there is no critical amount of time for the auxin to translocate to the rooting site. After auxins in the plant system operations, such as misting of leaves, can resume.
DISCUSSION
Water based rooting solutions, with auxin compounds such as IBA, can be applied to the leaves of plant cuttings. The solution enters the vascular system of the plant through open stomata. Auxins translocate polarly through the stems to the basal end of the cutting. IBA can be converted by the plant to IAA, by slow bio-transformation, and utilized by the cutting to make new roots at the basal end.
REFERENCES
(1) Davies P. J. (2004). Natural occurrence and functions, In Davies, P., Plant Hormones,
Biosynthesis, Signal Transduction, Action! Dordrecht NL: Kluwer Ach. Publ., pg. 5-6
(2) Van Overbeek, Gordon and Gregory, 1946, An analysis of the function of the leaf in the
process of root formation in cuttings. Am J of Bot. 33:100-107.
(3) Aloni, R., the Induction of Vascular Tissues. In Davies, P., Plant Hormones,
Biosynthesis, Signal Transduction, Action!, Springer, 2004, pg. 474.
(4) Friml and Zazimalova (2004). Auxin Transport, In Davies, P., Plant Hormones,
Biosynthesis, Signal Transduction, Action! Dordrecht NL: Kluwer Ach. Publ., pg. 438-9.
(5) Aloni, R. (2004). The Induction of Vascular Tissues. In Davies, P., Plant Hormones,
Biosynthesis, Signal Transduction, Action!, Dordrecht NL: Kluwer Ach. Publ., pg. 477.
(6) Epstein and Ludwig-Muller. Indole-3-acetic acid in plants: occurrence, synthesis,
metabolism and transport. Physiolgia Plantarum. 1993. Volume 88 issue 2, pgs 383-389.
(7) Epstein and Lavee, Conversion of Indole-3-butyric acid to indole-3-acetic acid by
cuttings of grapevine and olive. Plant and Cell Physiology.1984.Vol 25,No 5, pgs 697-703.
(8) Mitchell, J. W ., and Marth, P.C. 1947. Growth Regulators for Garden, Field and
Orchard. Chicago: Univ of Chicago Press. pg 34.